Feed supplement
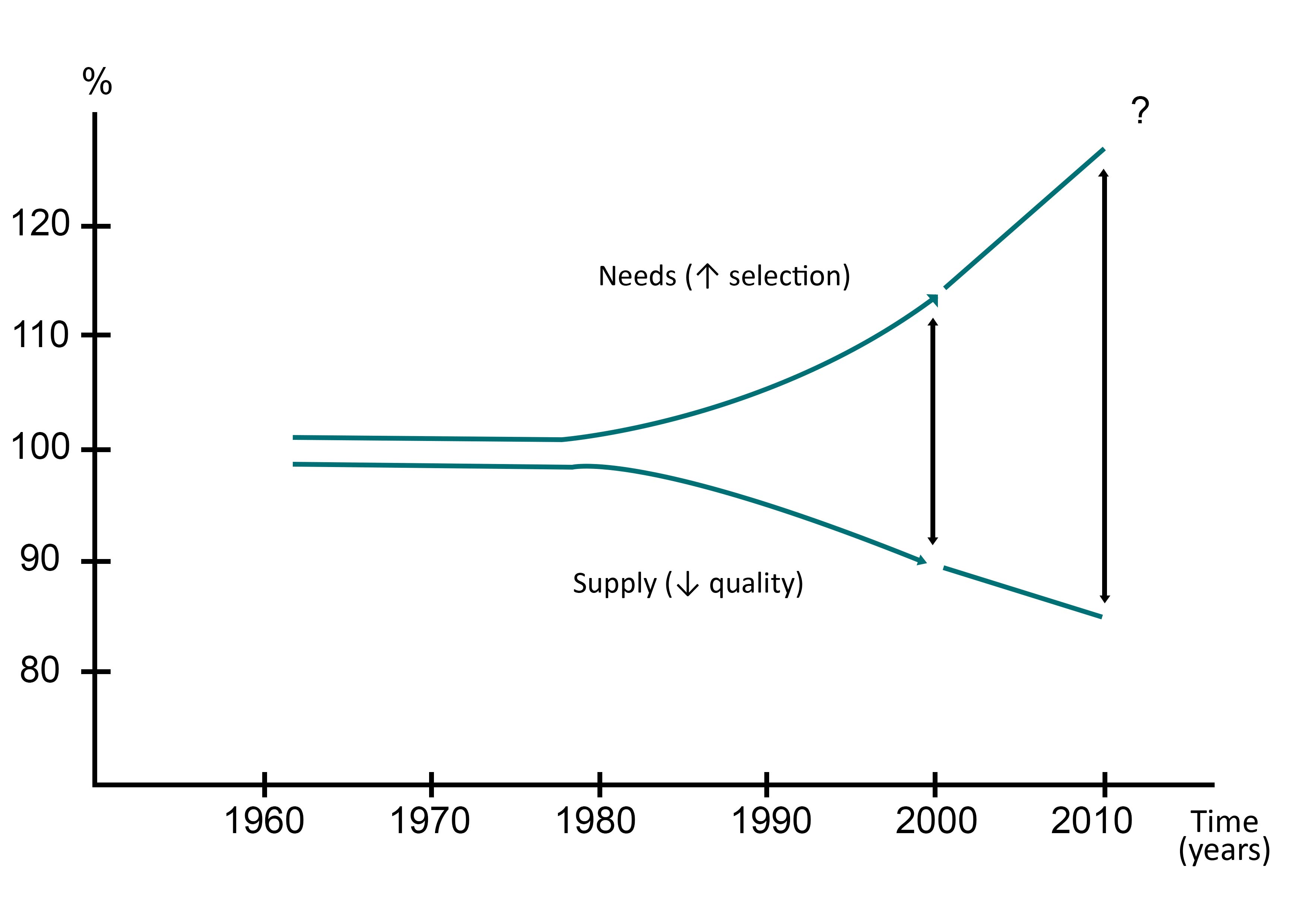
Practical implementation of feed supplement in a cattle farmIn cattle breeding, evolution of the current agriculture (monocultures, high productive breeds, deficient soils…) are responsible for a growing gap between the supply in trace elements and the needs of the animals (figure 8). This increases the risk of having deficiencies. Furthermore, because trace elements role in metabolic reactions is better known, it leads to more frequent diagnosis of deficiencies.
In this context, several tools allow the veterinarian to advice to his farmers management methods of trace elements deficiencies or excess within the farm.
Assessment of the feeding practices
Review of the ration as a whole
First, it is important to review the ration as a whole. Indeed, when food-born origin of a disorder is highlighted, major elements of the ration should first be reviewed such as energy, fibre, crude protein supply… (Arthington, 2003; Chantreau, 2017). In this instance, the following elements should be checked :
- Theorical rationing : that means assess the calculated supply for the ration, but also the approach system and its adaptation to physiologic standards and to livestock needs.
- Quality of the ration given : quantity of forages given and composition of minerals of these forages.
- The real quantity of ration ingested : depending on the dominance trends, on the parts of the ration “selected” by the cattle and uneaten.
- The part of the ration that is really used, that means which are the cattle performances compared to these technically expected with the ration they are given (Wolter & Ponter, 2013).
This same analysis should be done when trace elements deficiency is suspected. However, it is not very often carried out due to the price of the analysis. It is the comparison of the quantities supplied and the recommended daily allowances that allows to highlight deficiencies in the ration (Guerin, 2010).
Last but not least, it has been shown that the vast majority of the rations supplied to ruminants are deficient in minerals. It is even more important in the case of trace elements. Therefore, it is sometimes possible to directly have a look at the supplementation in minerals without analysing forages (Rollin, 2002)
The objectives of a mineral supplementation are :
- Fix a proven deficiency and reduce the associated clinical signs,
- Cover the optimum needs for animal health, welfare and productivity,
- Avoid excess (Rollin, 2016).
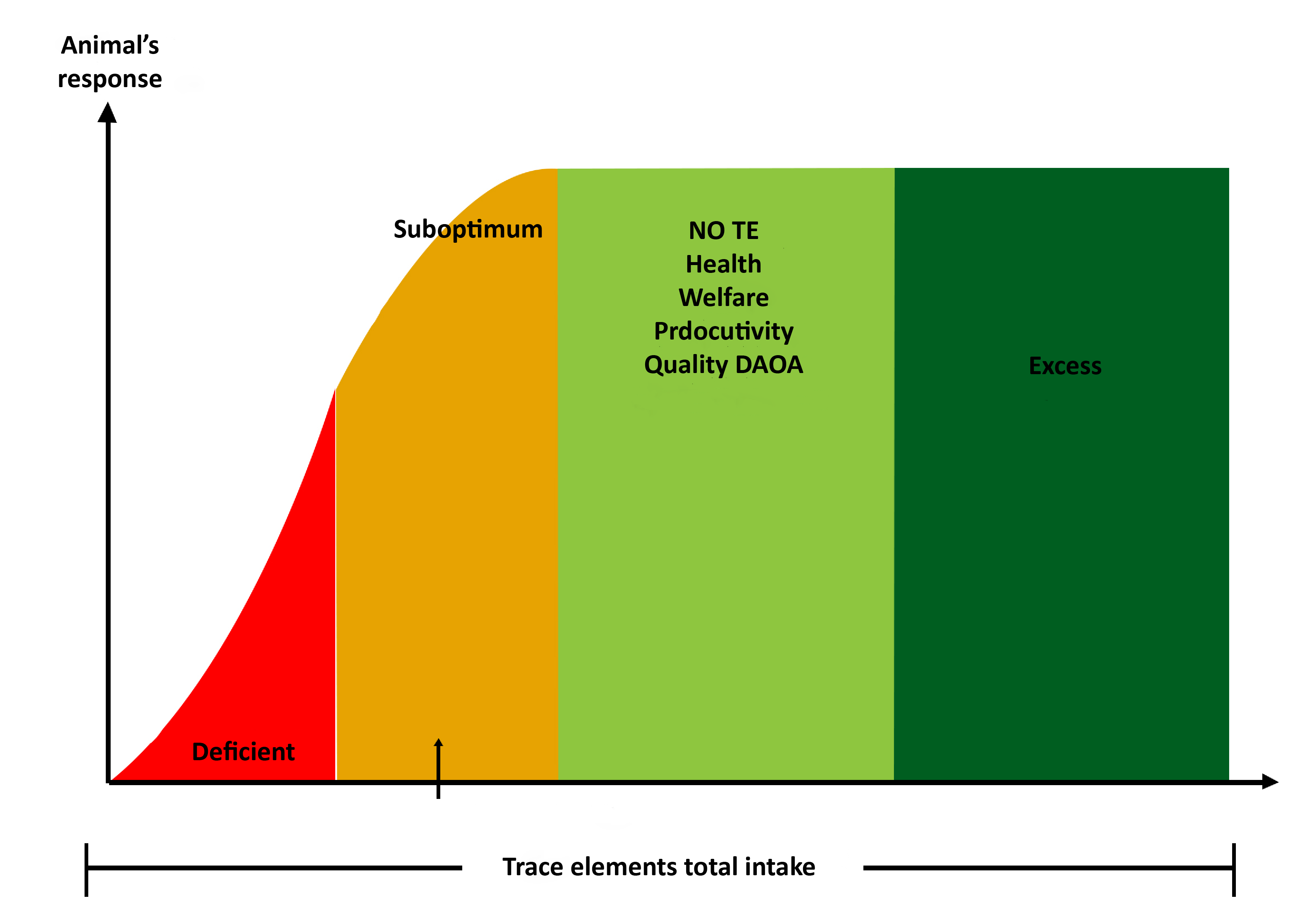
For this to happen, recommendations made by INRA (National Institute for Agricultural Research) exist. They have been issued in 1988 and reviewed in 2010. They include a safety margin in order to limit as much as possible to go over the toxicity levels (INRA, 2010). Indeed, some trace elements have a toxic action when they are absorbed in excessive amount. For instance, when Copper is in excess, a hepatic accumulation of the element happens followed by a sudden release in the blood circulation. This can lead to destruction of red cells and renal necrosis. Likewise, Iron, Iodine and Selenium are toxic as they are powerful antioxidants (Meschy, 2010). Therefore, the recommended intakes are between deficiency and toxicity thresholds for trace elements. They are often lower than the optimal amount for health, welfare and productivity, in order to keep a margin with respect to toxicity thresholds (see figure below).
It is however important to note that the recommended intakes vary depending on several parameters. Furthermore, as shown on the figure 10, they are relatively low compared to the production needs of the animals (Rollin, 2016):
Ces normes varient en fonction de certains paramètres et, comme le montre la figure ci-dessus, ils sont relativement bas par rapport aux besoins de production des animaux (Rollin, 2016) :
- Bioavailability of minerals considered (organic or inorganic form, possible competition or not)
- Animals productivity, stress
- Ingestion capacity (decrease at the end of gestation)
- Authors and reference bodies (INRA/NRC for example)
The recommendations have to be used as a basis for calculation of the supplementation with respect to the ration. Nevertheless, every farm has their own, depending on the ration used and its quality, especially regarding trace elements. It is also important to adapt the quantities supplied depending in the existence or not of certain substances in the ration, such as goitrogens products, thiomolybdates, nitrates, an eventual deficit in amino acids in the ration… (Pin, 2007).
There are two types of trace elements deficiencies :
- Primary deficiencies, which are linked to an insufficient intake in trace elements, due to a lack of supply, either in quality or quantity.
- Secondary deficiencies, which happen when there has been an intake of an element that inhibits the absorption of a trace element (Arthington, 2003).
Thereafter, it is necessary to have a look at practices that can lead to primary or secondary deficiencies.
Identification of poor absorption due to hazardous practices
Poor absorption hazardous practices correspond to practices leading to secondary deficiencies.
Secondary deficiencies in Copper are relatively common. Especially throughout competitions between Molybdenum, Sulphur, or Iron. The first two elements inhibit the absorption of Copper per collection and complexation of Copper. In this way they constitute molecules that are very less absorbed in the intestine and with a low bioavailability. When cattle graze on pastures where grass is too short, the ingestion of soil, including Iron that can represent up to 10% of dry matter, can be responsible for a Copper deficiency (Guerin, 2010). An excess in Sulphur, sometimes present in water for instance, also inhibits absorption of Selenium. Thus, usage of a well collecting rainwater, or the utilization of a fertilizer rich in Sulphur on crops are two hazardous practices for Copper and Selenium deficiencies (Arthington, 2003).
Any events responsible of alteration of the digestive tract can be responsible for a secondary deficiency in trace elements by decrease of the digestive absorption. Therefore, as an example, an untreated chronic diarrhoea can be responsible for deficiencies (Graham, 1991).
Identification of poor supplementation hazardous practices
Most of forages are deficient in trace elements. We therefore understand that lack of mineral supplementation represents a risky practice of having important deficiency (Rollin, 2002).
However, in most animal nutrition works, the majority of the studies concerns major elements of the ration such as digestible proteins, energy or nitrogen; then on calcium and phosphorus, and finally, few concern trace elements. Therefore, the recommendations in this area are often limited to one commercial product containing trace elements. However, this product should be affordable while being of good quality, which means being sufficiently bioavailable for cattle (Adams, 1975; Meschy, 2010).
Assessment of the supplementation types
Available galenics
Then, it is interesting to know which distribution mode is used and if the supplies are controlled, with an individual and measured distribution, or more random, with an ad libitum provision of minerals. The table below shows the different macroscopic shape available for farmers. When implementing a supplementation, and especially when choosing the galenic form, it is important to consider herd size, type of cattle (dairy/beef), and requirements in terms of time and labour for the farmer. Indeed, an indirect supplementation (drinking water, mixed in the ration) is often better than no supplementation at all, even if individual supplies are less precisely known (Meschy, 2010).
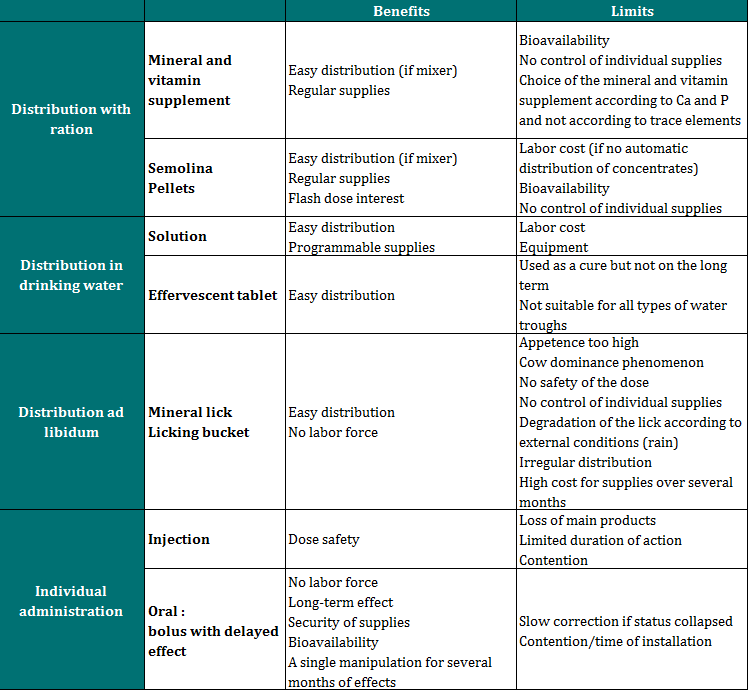
The type and frequency of trace elements supplies highly affect bioavailability. This latter notion corresponds to the proportion indeed active in the organism and metabolized compared to the quantity of elements absorbed. This corresponds to the valued part of the ration for trace elements.
Therefore, trace elements excretion increases when supplies increases. For instance, if we look at minerals distributed in the ration, the quantities supplied are relatively high in order to cover the needs, but frequency is low: only two times per day. Mechanisms of absorption are thus rapidly saturated, and a significant part of supplies are directly eliminated. Even if the quantity distributed seems to be enough to meet the animals needs, the quantity absorbed is too low – because of the type of supplementation. On the opposite, a quantity given during longer period, such as methods with continuous release, will be better absorbed by the animal and thus best used (Spears, 2014).
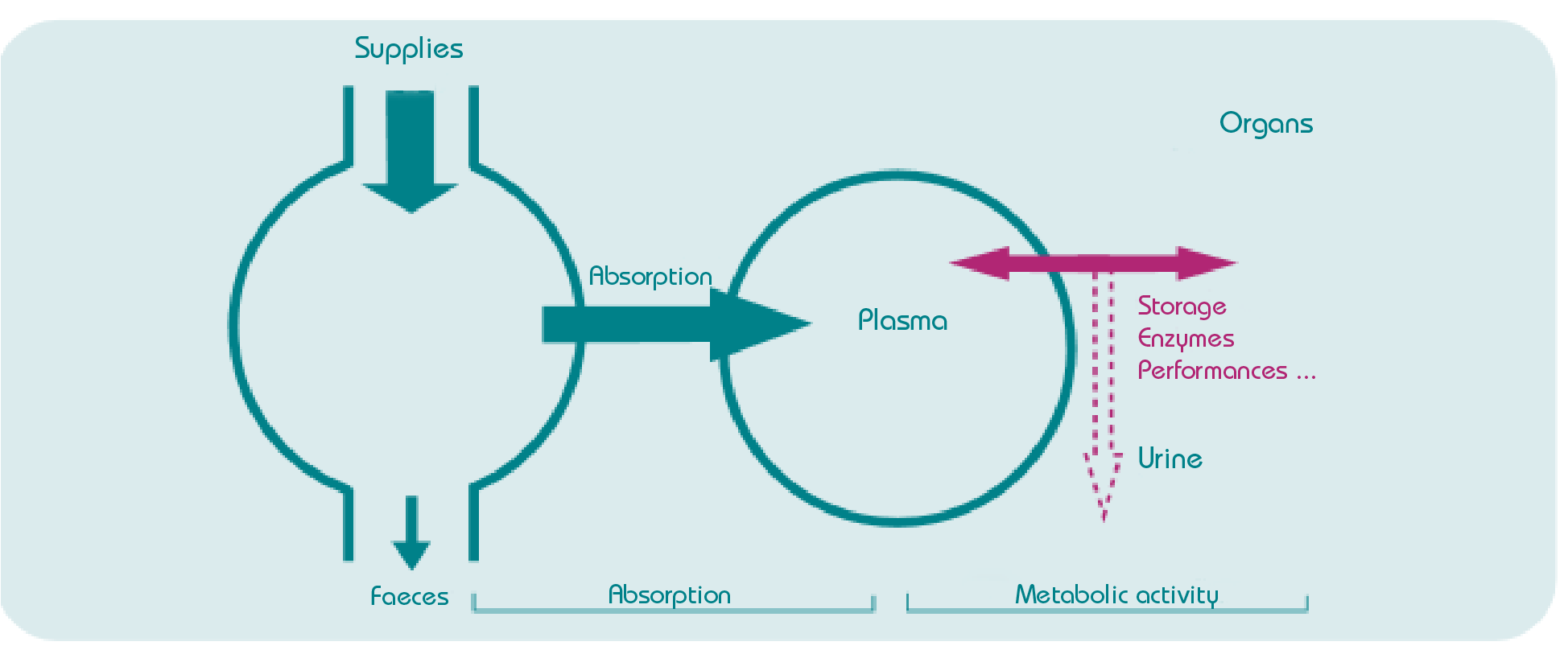
This way, we understand that the quantity of trace elements supplied in the ration is not a sufficient fact. Furthermore, bioavailability of an element implies that the latter has been absorbed as well as transported to the targeted tissue and that its action is completed. Yet, interactions exist between the different constituents of the ration. Furthermore, transit speed, digestive tract conditions and animal physiological phase are part of the factors influencing absorption of elements by the intestinal barrier. The definition of metabolic utilisation (figure below) allows to describe trace elements supplies, absorption, bioavailability, metabolism and elimination (Arnaud & Belleville-Nabet, 1995).
Solubility of trace elements is another major parameter influencing their bioavailability. The latter varies depending on the biochemical form given. It can also influence desintegration times of the delayed action mechanisms.
Chemical forms of supply
We mentioned the different macroscopic forms trace elements can have. However, even if this form is of great significance for trace elements bioavailability, it is important to take into account their chemical form. Indeed, sources of trace elements cannot be used the same way by the animals: they can be completely assimilated and used by the animal, partially, or completely eliminated, which makes their bioavailability vary (McDowell 2003).
Numerous chemical forms of supply in trace elements exist. They can be split in two groups: inorganic salts and organic salts. Since the late 1990s, a third category exists: the hydroxy trace elements.
Inorganic salts
It is composed of a mineral element and a carbonless ligand. These are the first forms put on the market, in the 50s. This group is made of :
- Sulphates (SO42-)
- Chlorides (Cl-)
- Carbonates (CO3-)
- Oxides (O2-)
Amongst these inorganic forms, sulphates are the most assimilable by the animals. Inorganic salts are widely used nowadays both to prevent deficiencies and as a curative solution when the deficiency is proved.
Rumen bacteria have a strong impact on carbonates and oxides deterioration and absorption. Purity, variable depending on the sources of minerals used, is an important element influencing bioavailability of an element. Moreover, bioavailability of organic salts car strongly be impacted by presence of antagonistic substances. Thus, this decreases metabolism of some elements. Less sensible to these interactions, the organic forms are often preferred as the bioavailability is then more important, despite a slightly higher price (Spears, 2013).
Organic salts
An organic salt is composed of a mineral element and a carboned ligand. It is either complexes, artificially synthetized, made of organic elements and metal ion, or complexes produced by yeasts. They have been developed in the 70s, in addition to inorganic forms, in order to enhance bioavailability. There are :
- Proteinates, complexes made of amino acids and metal ion,
- Chelates of only one amino acid with a metal ion,
- Polysaccharides complexes,
- Malate, fumarate and citrate, but are very little used in animal feed as very expensive,
- Selenium yeasts, only for Selenium.
The main objective of supplementation under organic form is to increase bioavailability of trace elements supplied. This can be done thanks to a covalent bond between the ligand and the mineral element. This bond would give to the mineral a better resistance in the digestive tract.
Hydroxyanalogue forms
A hydroxy trace element is composed of a mineral element, bonded to a hydroxyl goup and a chloride group by covalent bonds. These are usually elements that are not soluble in water. Therefore, they are non-hygroscopic and less reactive to sulphates. Furthermore, by improving bioavailability of trace elements, the quantity excreted in the environment through faeces are less important. Likewise, their low solubility to pH of the rumen, allows to reduce the impact on the rumen microorganisms. Indeed, cattle needs in trace elements are higher than those from the rumen microorganisms. However, if concentrations that are too high, especially in Zinc and Copper, they can be responsible for a deterioration of the rumen microorganisms ((Intellibond), 2013).
The type of salts used do not only determine trace elements bioavailability. For instance, supply of organic or inorganic forms determines the speed of disintegration of boluses from a galenic point of view. A bolus mainly made of organic salts will have a shorter disintegration time than a bolus manly made of inorganic salts.
Therefore, the idea for the manufacturer is to find the right balance between the disintegration time/desired length of action and the composition.
Assessment of the trace elements status of the animals
To assess cattle’s assimilation of trace elements of their ration makes it possible to assess the effective intake of the ration. The status in trace elements can be obtained through different samples: blood, milk, saliva or animal tissues.
Blood sample
Blood status in trace elements can be obtained in several ways:
- Direct, by measuring the plasmatic concentrations of the elements,
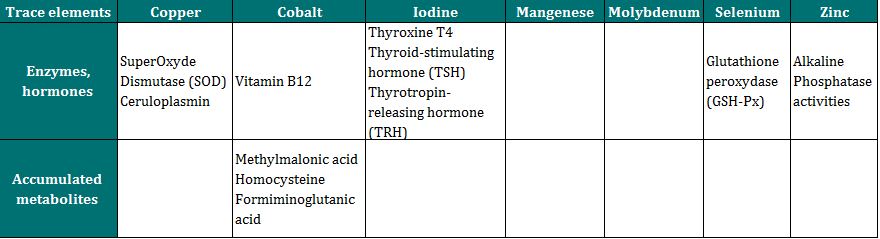
- Indirect, by assessing the activity of certain enzymes or hormones of which the functioning and concentration depend on specific trace elements,
- Indirect, by measuring concentration of certain metabolites which accumulate during deficiencies (table below).
Indirect measurement elements of the status in trace elements (According to Herdt & Hoff 2011)
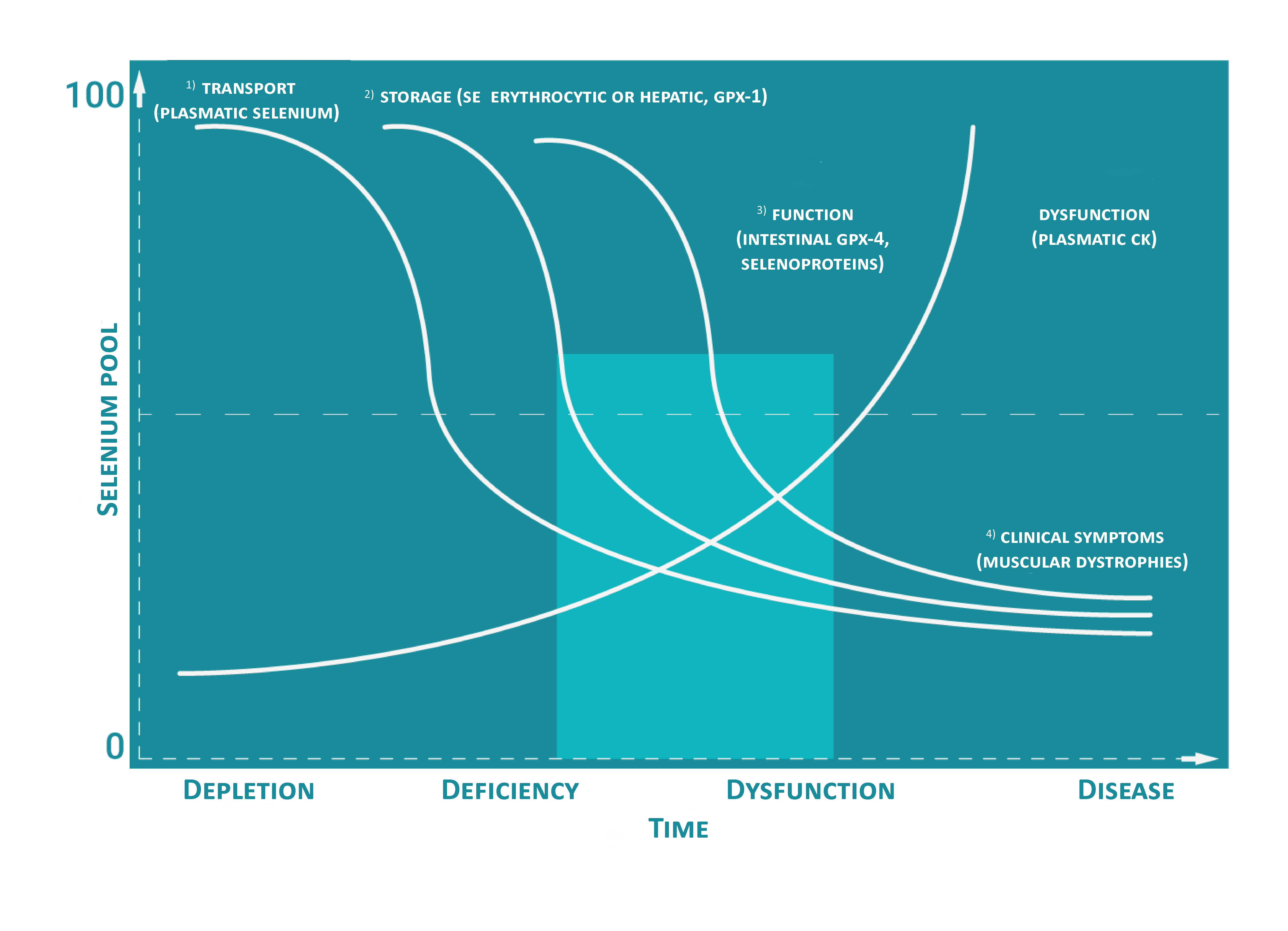
Direct measurement is not possible when there are clinical signs, which means the deficiency is quite serious (figure below). Nowadays, breeders are more and more willing to identify deficiencies when those are subclinical and before they have an economic impact. These indirect measures are no longer enough. Furthermore, measures of this types require a different technic of analysis for each trace elements. This is the reason why the price is significantly higher as if we were measuring the total plasmatic concentrations in trace elements.
Measurement of plasmatic concentrations in trace elements is the reference method the most used as the easiest to technically execute. This is also a standardised method and thus easy to repeat. Moreover, the lastest technical innovations, such as dosage by plasma torch couples with mass spectrometry (ICP/MS), made it possible to reduce farmer’s costs, obtain rapid and precise results (Chappuis & Poupon, 1991; Lumet & Negriolli, 2007). Howevere, it has been shown that plasmatic concentrations in trace elements could be influenced by feed intake, homeostasis, physiological stage or age (Herdt & Hoff, 2011).
When the objective is to determine a plasma profile of livestock in trace elements, it is relevant to select animals at random in herds, but should represent best the herd. Indeed, it increases the impact of the predictive values of the analyses and the random selection makes it possible to reduce individual variations, especially those related to the physiological stage (Herdt, 2000; Siliart, 2014). However, it is important not to sample them when they are too young, less than 2 months, neither in the month before or after calving, as the variations in minerals during theses periods are very high and not representative of the global status of the herd. Moreover, the sampled animals have to be clinically healthy, as trace elements status are strongly affected by different diseases – for instance, steric concentrations in Copper increases during the inflammatory process (Herdt & Hoff, 2011).
The optimum number of animals to sample corresponds to 10% of the total number of cows of the herd. But it also depends on the objective of the analysis: estimate the average concentrations in trace elements of the herd, assess a deficiency prevalence or highlight it. The minimum number of animals to sample varies from 4 to 12 depending on the authors (Herdt & Hoff, 2011; Kincaid, 1999; Oetzel, 2004). In order to lower the costs, sometimes pooled samples from several animals can be taken. Then, between 5 and 20 animals can be sampled depending on the authors, and group average values can be compared to reference values of this group. However, when dosing glutathione peroxidase for the assessment of Selenium, the number of individuals to sample would be of 5 maximum. Beyond, the obtained value for the mix would statistically be too different from the average value of the individuals (Pitel, Besnier, Reisdorffer, Defontis, & Richard, 2014). However, the use of pooled samples to assess a herd’s status is subject to controversy. Indeed, pooled blood sample may hide heterogeneity of status between animals. Moreover, just one extreme value can significally change the results. Thus, only very high or very low results could be interpreted (Guyot & Rollin, 2007; Siliart, 2014).
Finally, during blood tests, it is relevant to choose the right sample tube. Indeed, tubes made of glass or those with a rubber cork are at risk to contaminate the sample, favouring hemolysis or by artificially increasing Zinc level. It is recommended to sample on EDTA K2 tubes, specifically treated on the cork to make trace elements dosage possible.
These tubes are supplied by Vétalis to all veterinarians who request them.
Sample of tissus
These samplings allow an access to different trace elements stocking organs. Therefore, liver biopsy gives a direct assessment of Copper, Iron and Cobalt stock. Even though this sample is relatively easy and quick to do, it is more expensive and slower than a blood sample. This is why it is not often used in Europe.
During a longer fast, stress, inflammations or infections – even starting – quantities of trace elements stocked change. This happens before apparition of any clinical sign or any variation of plasma concentrations (Suttle, 2010). This is why, when sampling is possible, biopsies can be worth to be carried out – even more in clinical context (Herdt & Hoff, 2011). Finally, chronic exposure to high doses of minerals causes their accumulation in the stocking organs, quite often without increasing their plasma levels. Therefore, biopsies make possible to objectify intoxication before clinical consequences appear (Auza, 1983).
Milk sample
For Iodine and Selenium, excreted amounts in the milk reflect the short-term status of the animal. The analysis can be done either on one individual or on the milk from the tank. However, it is important to make sure that the contaminated milk by erythrocytes hasn’t been put in the mixed milk. This would distort the Selenium value (Guyot, Saegerman, Lebreton, Sandersen, & Rollin, 2009).
However, Iodine cannot be sampled on the colostrum as it contains three to five times more Iodine than the milk. Moreover, when using iodine-based products, this sampling is useless as the products artificially increase the iodine level measured (Meschy, 2010).
Other samples
Urinary and hair profiles could seem interesting at first sight because easy to do for many people. However, they are not the most relevant samples. Indeed, urinary concentrations in trace elements are not correlated to blood concentrations: when feed supplies increase, urine elimination also increases. Furthermore, urine excretion depends on the trace element and on the intake type. For instance, Selenium renal excretion is increased when the latter is supplied in the inorganic form compared to an organic supply. In general, when there is no kidney damage, trace elements in urine come from an excessive supply or a sudden weight loss (Guyot & Rollin, 2007; Siliart, 2014).
Hair concentrations highly vary depending on the colour: black hair is richer in Zinc, red hair is richer in Copper. Moreover, these concentrations are lagging indicators of a possible deficiency. Finally, hair is very often contaminated by the external environment (when friction against the fence for example) and dust. Therefore, hair concentrations in trace elements depend more of the hair colour, the age of the animal and the external environment than of his status (Meschy, 2010; Siliart, 2011, 2014).
Thus, urine or hair samples are not suitable for assessing the trace element status of cattle.
 Contact
Contact Export
Export